Hi WCR fans, I have seen this for as long as I have done residential but do not know what puts it there.
I’m talking about when you remove a screen with those leaf spring style especially. Then you find the track filled with dried grass clippings, or something that looks like it.
It is stuck in the leaf spring on the screen and in the track of the vinyl window frame as well. Sometimes it is so thick it falls out and if the window is raised it blows inside the house. Like I said it looks like dried grass clippings, but there is no way it could float there on the breeze when the lawn is cut.
[B]Does anyone know what is is for sure and how it gets there? [/B]
I’m really curious!
Matt
[/IMG]
[/IMG]
Some type of insect, I thing it may be grasshopper larvae, we run into it on triple track storms a lot.
Sent from my iPhone using Tapatalk
I’ve seen small birds stuffing grass clippings in tracks. Don’t know why but they were doing it at a few houses we clean.
I think it may be the wind. I’ve always noticed the worst on the ground floor and when the window is overlooking grass.
I just did a job the other day and I was wondering the same thing.
I’m almost sure insects are the culprits. Sometimes they are so crammed on double-hungs that the sashes can barely be moved.
We get it real bad around here with casment windows, bugs will build nests on the top of the window, between the frame and the window unit.
You’re all wrong – it’s magic!
Hey Matt…
Great video about your grand kids…Your a lucky Man !
In my limited estimation I’d have to say it’s… entropy !
Take care Matt and always be safe !
Dange
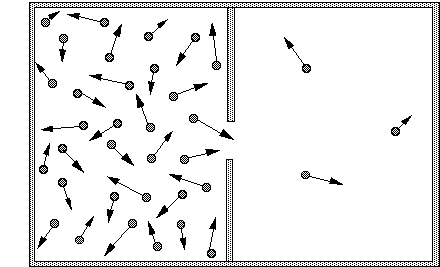
Entropy increase: the box with two compartments
Fig. : a box containing air molecules, divided in two compartments with a hole in the dividing wall. The arrows represent the direction and speed of movement of the molecules. Since there are many more molecules in the left compartment, there will also be many more molecules crossing the divide from left to right.
The second law of thermodynamics states that in an isolated system the entropy will increase. In the statistical definition of entropy according to Boltzmann, this means that the system will evolve to its most probable state, that is, the one with the most homogeneous probability distribution. Let us explain this idea with a classical example: a closed box with two compartments (Fig. 1). The left compartment contains air, the right one is empty. Suppose that we make a hole in the wall separating the two compartments. An intuitive description of what happens is that air will be “sucked” out of the full compartment into the vacuum of the empty one, until both compartments contain equal amounts of air.
This effect is easy to understand when you look at the individual air molecules in the two compartments. The molecules are continuously flying in all directions, colliding with each other and with the walls of the compartment. A molecule bumping against the central wall separating the full compartment from the empty one will be reflected, and move back towards the middle of the full one. However, if the molecule would land on the hole, rather than on the separating wall, it would not be reflected but continue its journey rightwards into the empty compartment. Since many molecules are thus continuously flying from the left to the right, the number of molecules in the right compartment will quickly increase. These molecules too will collide and be scattered in all directions. Some of them will move to the left, into the hole, and thus go back to the left compartment. However, as long as there are less molecules in the right compartment, there will also be less that move through the hole from the right, and thus rejoin their erstwhile companions. As long as the concentration of molecules on the left is larger, there will be more movement from left to right than from right to left. Only when the concentration of molecules in the two compartments is uniform will there be an equal flow through the hole in both directions. This configuration where the distribution is homogeneous is the one with maximum entropy.
Now, it is in principle possible that by some coincidence more molecules would move to the left than to the right. This creates a difference in concentration between the two boxes, and therefore a decrease in entropy. However, since the amount of molecules in either compartment is so astronomically large, a very large number of them must move “countercurrent” to produce a noticeable difference. The probability that such a vast number of molecules would all move together in the same direction is vanishingly small. It is so small, in fact, that we can assume that it will never happen. Because of the law of large numbers, the larger the number of coincidences needed for an effect to occur, the smaller the probability that the effect will occur. And with numbers as large as the number of molecules in a gas, the resulting probability is as close to zero as you can practically get. Therefore, although entropy could decrease spontaneously, that is so improbable that the non-decrease of entropy has gotten the status of a law of thermodynamics. While we cannot predict the movement of the microscopic particles, the evolution towards homogeneity on the macroscopic scale is perfectly predictable.
Generalization
Thanks on the Grand kids, and thanks to your reply, the next customer who ask what causes that, I now have a simple answer!
“who knows”
“So keep calling me every 6 months to clean it out of there for you.”
Interesting, I look each time to see if I can find any life form that is caught in the act, but only find dead bugs if any at all.
That,s a thought, after all the customer wants us to make it disappear!
Once a Brother Window cleaner and I were working together down in SoCal, he was doing the inside and I was laddering the outside.
We both came to the same window at the same time on the second floor. I was working on the ladder and ask him to take out the screen for me.
Well he was having trouble getting it out so he put his head close to the screen and looked up while working the screen to get it out !
When he popped it out, there was hundreds of earwigs all crammed in the frame between the frame and screen and they all came crashing down on him, onto his head and neck, into his mouth and down his shirt…They were all alive and running all over him !
He freaked out dropped the screen, ripped off his shirt and was spitting out the window. I was laughing my butt off screaming at him that he was slowing me down !
Dangerous
Sounds… dangerous.
I have my money on ants, or magic ants!!!
I was wrong ![]()
And the answer is : Out of My Head » grass window frame - Thoughts on Technology, Science, Gardening and Exercise
Yeah, the earwig will travel into your ear and then dig through the ear canal and will reach the center of you brain and lay eggs that will hatch in the hundreds and they’ll leave through hundreds of directions and then leave that many holes there, the opposite of mad cow disease ! Yikes…
Dangerous Dave
Steve-o your an excellent internet researcher !
I blame it on the recent infestation of these little bastards…
Apparently most of the bat population has died off in NJ due to white-nose fungus (no, I didn’t make that up lol). Because of that, these little emmereffers are EVERYWHERE! I found a live one in January in a track, couldn’t believe my eyes!
On the same subject, found a relatively large dead fern leaf half-in-half-out of a screen the other day. Could not for the life of me figure out how it got there. Nature works in strange ways
Thanks, I knew we could solve the mystery if I asked the WCR Community! I also figured we would get some interesting replies.
Thanks for the input!
I think that, I will now use my track brush more often instead of my bare fingers!


